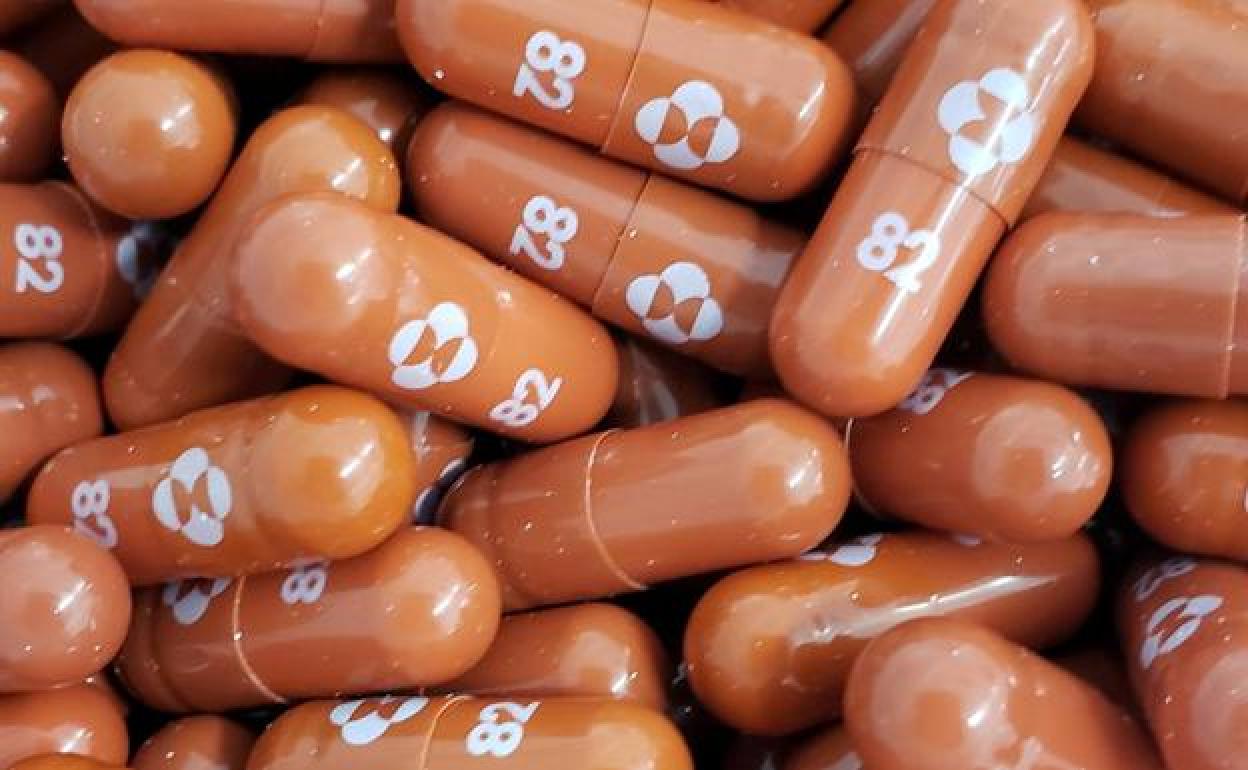
The European Medicines Agency approves the Covid-19 pill from the US laboratory MSD
Molnupiravir is intended for people with mild or moderate coronavirus who have at least one risk factor for developing a severe case of the disease
R. C.
Thursday, 4 November 2021, 18:55
The European Medicines Agency (EMA) has this Thursday, 4 November, approved the use of molnupiravir, the tablet treatment for Covid-19 from the US laboratory MSD.
Just hours earlier, the United Kingdom also gave the green light to the use of the drug. Molnupiravir was authorised by the British regulator, the MHRA, for people who suffer from a mild or moderate coronavirus and have at least one risk factor for developing the disease in a serious way such as obesity, age over 60 years, diabetes or heart disease.
Antivirals, such as molnupiravir, work by reducing the ability of the virus to reproduce, thus slowing down the disease. The use of the drug can be twofold: both to prevent those infected from suffering serious symptoms, and to prevent those who have been in close contact from developing the disease. If given to patients in the days after a positive diagnostic test, it reduces the chances of hospitalisation by 50 per cent, according to a clinical trial conducted by MSD.
480,000 treatments
The British Government announced in October that it had ordered 480,000 treatments of molnupiravir. The United Kingdom has counted more than 140,000 deaths from coronavirus and is currently registering an increase in infections, with about 1,000 new hospitalisations a day. Although these figures are lower than those recorded at the peak of the pandemic, the authorities fear that the health situation will worsen with the arrival of winter.