Dani, the little boy from Malaga with a rare disease who is undergoing pioneering therapy in Spain
His mother Luz María Blanco told SUR: "We are hopeful that Dani will walk and talk" and has thanked staff at the city's Materno Infantil hospital, as well as the Columbus Foundation and the San Juan de Dios Hospital in Barcelona, where the operation was carried out on 9 September
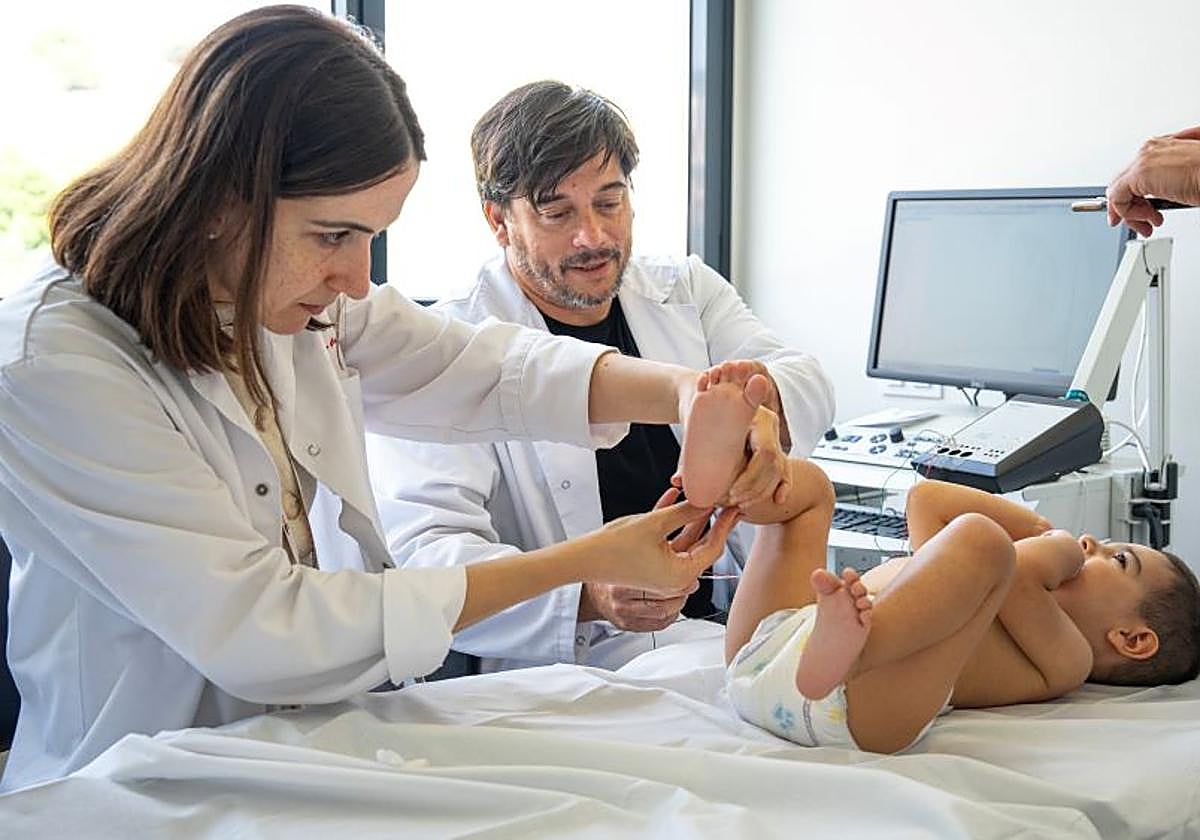
Two-year-old Dani from Malaga has undergone pioneering treatment at the Hospital Sant Joan de Déu in Barcelona (HSJD), for the ultra rare hereditary disease he has known as hereditary spastic paraparesis type 50 (SPG50). It is a neurodegenerative disease so rare that there are only around a hundred diagnosed cases in the world.
Dani is receiving an experimental gene therapy being used for the first time in Spain, which brings new hope for other children with the disease. As the child's mother, Luz María Blanco, a resident of Mijas, explained to SUR: "We are hopeful that Dani will walk and talk".
The treatment is possible thanks to collaboration between the hospital and the Columbus Foundation in Valencia. SPG50 is, as reported by the Hospital de San Juan de Dios (HSJD) in Barcelona, "a serious disease that causes delays in motor and cognitive development, affects language, causes microcephaly and gradually leads to severe muscle spasticity" (which causes the muscles to be tense and rigid).
100 cases
approximately there are diagnosed worldwide with hereditary spastic paraparesis type 50 (HSP50).
"The genetic alteration is corrected and the virus is stopped", says Luz Blanco, who says that her son is responding well to the experimental treatment and she hopes that it will stop progression of the disease. "We are very happy and grateful, because of course, for parents to receive a diagnosis, when their little boy has just turned two, that he has an ultra rare degenerative disease that is going to get worse, that is very hard". Luz explains that "the intervention consists of introducing the drug into the spinal cord, using a virus vector to introduce a healthy gene that replaces the bad gene".
Intervention
Dani received a dose of the drug Melpida on 9 September, the only existing gene therapy for this disease, making him the youngest patient treated in Spain and one of just nine children treated around the world. The intervention was performed intrathecally (in the lower part of the spinal column) and with imaging support and was successful and without adverse reactions.
Dani only needed to spend two days in hospital and is currently improving under the supervision of specialists at San Juan de Dios and the Materno Infantil in Malaga city. "With this intervention the genetic alteration is corrected and the disease is stopped," Luz explains, although as the results are seen in a period ranging from six months to a year, they will have to wait to see just how effective the drug has been. "We see him more connected and more agile in terms of motor skills, but we don't know if it could be because of the physiotherapy, equine therapy sessions and the speech therapist."

Zoom

It was the Columbus Foundation, whose aim is to facilitate access to more advanced and effective therapies for children with cancer or rare diseases, which took Dani to the Barcelona hospital in May 2025. It is a reference centre in Spain for patients with this disease through its neuromuscular pathology unit.
The doctors at the Barcelona hospital contacted Dani's referral doctor at the Hospital Materno in Malaga, assessed the case based on laboratory tests, magnetic resonance imaging and a clinical interview with the family, in order to confirm the compatibility of SPG50 and the suitability of treatment with experimental gene therapy.
Urgent treatment for Dani: every day counts in the fight against his rare disease
Dani's referral doctor at the Materno Infantil, a specialist in neurology, gave the family hope. "He told us that there was a possibility of treatment, he contacted a hospital in San Sebastian and the United States, where they had the drug, and began the process for Dani to be treated. That was in April and within five months, Dani was treated.
"We look to the future with hope".
"We look to the future with hope, so far this therapy has given very good results," Luz says, while thanking the Columbus Foundation, which has paid for the treatment which comes from the USA. "They assumed the cost and they continue, because there are check-ups every three months," she says, adding: "This has gone well in other places, we spoke to another family who told us that we had to be prepared because it is very hard, as children are often unwell after the operation, but Dani was crawling in the ICU the same afternoon, he was discharged the next day. His attitude said it all."
"We have full confidence in the improvement, we are excited and hopeful that he will walk and talk. These children do not walk independently, but with walkers and some of them are unable to speak. We are hopeful that he will walk without a walker and talk." Luz stresses that the aim is for the child to be as independent as possible and therefore lead "as normal a life" as possible. These interventions tend to improve patients cognitively, Luz explains.
The development and administration of the drug Melpida has been possible thanks to a strong network of international collaboration: the drug was developed at the Southwestern Medical Center of the University of Texas, driven by the exemplary fight of Terry Pirovolakis, the father of a Canadian child diagnosed with the disease, who has developed the therapy through a non-profit company (Elpida Therapeutics) in North America. The production has been carried out by the Basque biotech company Viralgen and the Columbus Foundation has managed the logistics and family support.
Early diagnosis is key
Dani will continue to receive treatment via immunosuppressants at the Barcelona centre, which will be progressively withdrawn after the third month if the evolution is positive. He is now in Malaga, where he is undergoing check-ups and will only have to return to Barcelona to undergo some tests. We will have to wait to see how he evolves.
Neuropaediatrician warns of challenges in early diagnosis of SPG50 disease
"The symptoms present in patients with SPG50 can manifest themselves in other neurological conditions, which often makes it difficult to reach an early diagnosis, leading to a delay in the start of treatment," says Laura Carrera, neuropaediatrician at the Neuromuscular Pathology Unit of the HSJD.
As well as Dani, four other Spanish children have been treated thanks to the Columbus Foundation, in reference centres in Spain and abroad. In total, nine patients have received this gene therapy and all have experienced cognitive and motor improvements, especially when the intervention is performed early.